THE CHEMISTRY OF MINERAL TANNAGE
Delimed pelt- outcome of pre-tannage- is still raw material. When moist it is soft and pliable. Theaim is to make it durable, soft, porous opaque, together with stability over a wide range of physical and chemical conditions (pH, T, humidity).
The tanning agent must be able of crosslinking the molecules of collagen (has to be multifunctional). Degree of crosslinking needs careful consideration: if too much crosslink, than the product is harsh and brittle (mobility of fibrils are restricted).
Apart from introducing a limited number of crosslinks, a tanning agent should not at the same time lead to undue fibril modification (reduction in fibril length or solution of protein material).
In addition to number of crosslinks introduced by tanning, their general character is of importance. Could be: H-bonds, ionic bonds, covalent bonds.
CHROMIUM TANNAGE
Chromium: atomic number 24, wt 52, configuration [Ar]3d54s1.
common states Cr+3 and Cr+6( ie. CrO4-2)
Chromium tanning is between collagen and Cr+3. 6 coordination positions (octahedron) are present, and stereoisomers are possible. In solution, chromium III nitrate is tought to give a complex ion of the form [Cr(H2O)6]+3. 3NO3–. Upon storing, color changes from violet to blue, to green. Two primary stages (may occur simultaneously):
1) release of H+ from the hydrated cation to give a salt
[Cr(H2O)6]+3 . 3Cl– -> [Cr(H2O)5 OH]+2 . 3Cl– + H+
this reaction accounts for the acidity of solutions of Cr salts (ie. chromium chloride has pH<2). Addition of mineral acids reverse this reaction but addition of alkali promotes it.
2) entry of the anion into the complex with displacement of H2O
[Cr(H2O)6]+3 . 3Cl– -> [Cr(H2O)5 Cl]+2 . 2Cl– + H2O
in presence of neutral salt ie. KCl, anionic complexes may be formed.
[Cr(H2O)2Cl4]- K+. chloride ions held in complex are not precipitated by addition of silver nitrate.
Anions vary in ability to enter into complexes. Usually the stronger the acid formed by the anion the less tendency it shows to form complexes.
Other reactions:
Olated polynuclear complexes: here the metal ions are linked through OH bridges (the OH groups are not free for titration by acid -resistant to de-olation).


With increasing hydroxyl content or basicity there is a tendency to insolubility (olation).
Basicity: it is defined as percentage fraction of OH combined with chromium relative to the hydroxide Cr(OH)3 , which is 100 % basic. Therefore [Cr(H2O)5 OH]+2 is 33 % basic.
Oxolation: conversion of OH groups to oxo groups

these are even more resistant to acids.
Mixed bridge formation: takes place when other anions are present.
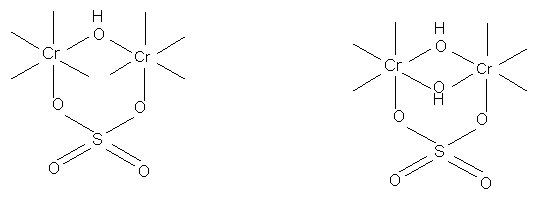
Masking and masked solutions:
Anions which are firmly held in a complex retard penetration of OH ligands. Therefore they may prevent formation of large olated and insoluble complexes. This action is known as masking. Entry of masking agents into the chrome complexes in solutions of basic chromium sulphate appears to depend on:
a) relative amouns of masking agent and Cr
b) absolute concentration of Cr
c) presence of other competing ligands(sulphate, chloride, hydroxyl)
d) whether competing ligands are added together or separately
e) pH
f) T
g) time
h) whether ligand is added as free acid or salt
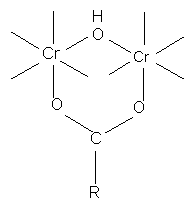
with monocarboxylic acid

with dicarboxylic acids
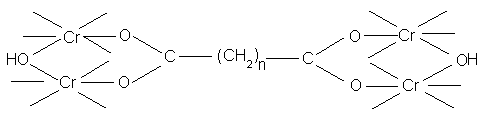
if less than 2 carbons separate COO groups (ie. oxalic acid chelate ring structure of extreme stability, therefore use of oxalate ions in quantitative estimation of Cr ions)
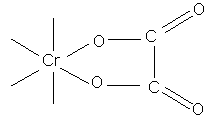
tartrate complex
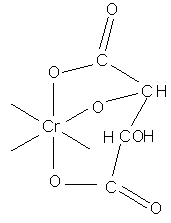
Masking action of geometrical isomers (cis/trans) maleic and fumaric acid:
Addition of sodium fumarate to a basic chromium liquor leads eventually to formation of an insoluble polymer.

whilst maleic anions give

When used under controlled conditions, the chain forming dibasic acids are of great technical importance, eg. Where large amounts of fixed chromium are needed to fill the loose flank regions of a hide.
Rate of reaction will depend on the nature of the ligands already present in the chrome complex. If large amounts of very stable masking ligands such as oxalate ions are present, no tannage will occur, since these can not be displaced by carboxyl groups (a small amount is O.K.). When using masked liquors in industrial level, the aim is to prevent excessive and rapid reaction in the grain and flesh regions of the pelt, allowing adequate amounts of Cr to penetrate into central regions where collagen carboxyl groups can react with complexes.
Pretannage operations of liming and deliming leaves the pelt collagen at a pH 5-6. Not far removed from the isoelectric condition.
With basic chromium sulphates, reaction with the pelt would be very rapid and lead to overtanning of outermost surface unless special precaution is taken.One approach is to used masked tanning salt. Another approach is to discharge the carboxyl groups of pelt collagen by back titration with strong acid. Unionized carboxyl groups are inactive in forming complex with the Cr (tanning action completely prevented) and hence penetration of the pelt by the chrome liquor may be achieved. The subsequent addition of alkali or highly basic Cr salt raises the pH value and tannage takes place. Excessive swelling of the pelt by acid is prevented by adding neutral salt to the pickle liquor (Balanced conditions require skill).
Particle size of Cr complexes are also of importance. It is thought that polynuclear complexes of 2 to 7 Cr atoms are present in solutions of chromium sulphate of basicity 33-50. It was found that at 40 % overall basicity the addition of carboxylic acid masking agents could increase the particle size twofold. At higher basicities, insoluble masked complexes and aggregation takes place. Particle sizes are obtained by rates of diffusion of complex ions.
The chromium sulphates used in leather industry are predominantly cationic at concentrations normally (2 % Cr2O3 solutions ) used. Concentrated stock liquors (11-15 % Cr2O3 ) and dry powders (25-33 % Cr2O3 ) when freshly diluted may be non-ionic or even anionic in character. Conversion to cationic character is always favored by aging of dilute solutions. With more easily displaced sulphate ligands, the chromium complexes will revert to the cationic form more rapidly than when organic anions are involved.
Little is known, of the rate of reaction between particular chromium complex ions and competing ligands, whether in solution or in actual tannage of pelt. Following reaction sequence suggested:
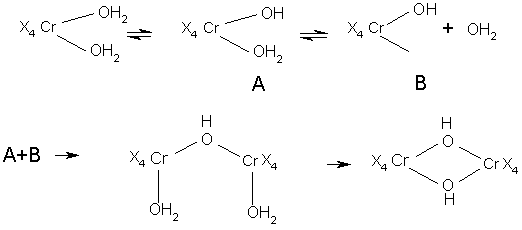
Conditions are likely to be most complex, overall reaction rate being influenced by factors such as:
a) compactness of pelt structure (affect diffusion rates)
b) sizes of Cr complexes
c) rate of over-all-coordination
d) pH
e) T
f) relative concentration of reactants
g) nature of ligands in complexes
h) nature and addition sequence of competing ligands
ZIRCONIUM TANNAGE
Basic Zirconium salts have definite tanning action with a shrinkage temperature of 90-95 0C. Such leather is of firm, full substance and has excellent white apparence. As with chromium, the sulphate, rather than the chloride, is the prefered starting material for making the tanning salt. It would find wide application provided high cost of tannin salts could be diminished. Large amounts of zirconium salts are needed (more than double the amount required for chromium tannage) to produce satisfactory leather, this is particularly with solutions of 33 % basicity and may be due to large particle size involved. Zirconium tannage most probably is a salt formation involving anionic zirconium complexes and basic groups in collagen.
Zirconium is Zr+4 and has coordination number 8. Neither the zirconyl group, Zr=O, nor the Zr-Zr group is found in solution.

Single OH s may be relaced by acid residues or carbonate residues. Formation of insoluble zirconium compounds starts when NaOH is added already at pH=1.5.
Masking: monocarboxylic acids have no effect, hydroxy acids show masking effect. Mechanism is thought to be a ‘multipoint attachment of zirconium to collagen.
1)Binding of anionic sites of zirconium complexes to amino groups
2)Polar binding of cationic sites of complexes to carboxyl groups
3)Covalent bonding of neutral sites and oxgen atoms of nonpolar carboxyl groups of collagen.
ALUMINUM TANNAGE
For a long time aluminum tanning has been known as tawing, with a paste containing NaCl, egg yolk, flour, water and potassium alum(white crystalline solid containing aluminum sulphate, potassium sulphate).Mechanism of tanning is expected to be like that of cromium but with much less stable complexes.
One reason for the pre-emminance of chromium as a tanning agent is its ability to form stable basic sulphates.Al+3 can not bind sulfate residues inside the complex formed.
Al2(H2O)12 (SO4)3 -> 2H+ + SO4-2 + [Al2(OH)2(H2O)10 ] +4 + 2SO4-2
Work on aluminum tannage has shown that reasonable stable basic salts may be obtained with aluminum sulphate or chloride, by introducing organic acid ligands such as tartaric, oxalic or gluconic acids. They attack aluminum complexes at pH= 4-5. These have only little tanning action, the rise in shrinkage temperature being of the order 15 0C, compared to 50 0C expected from chromium tannage.The low tanning property is due to instability of their inner sphere. Alum salts may be used in conjunction with other tanning agents to obtain specific effects (filler, dye precipitant to give intensive shades). In fur and wool-skin, pre-treatment with aluminum salts ensures minimum swelling during chromium tannage.
As a rule aluminum tanning is done in floats of zero basicity, at high concentration, in presence of NaCl (to prevent swelling), at pH about 2.5-3.5.
Aluminum tanned leathers are more resistant to hydrolysis after aging. They are to be finished after 3 months of tanning.
Aluminum tanned leathers are white, soft extensible but they are sensitive to water and high temperature (highest Ts achieved 75-85 0C).
TANNING WITH IRON SALTS
Leathers tanned with Fe salts are thin, brittle and do not resist aging. Oxidation and weak binding of complex to the hide is the reason for lack of resistance to aging.
When pH of tanning is 1.8-3.0, Ts from 65 to 90 0C may be obtained.
TITANIUM TANNAGE
Titanium may be used alone or with Cr and Zr compounds. Ammonium titanium sulfate
TiO2 SO4 (NH4 ) 2 SO4 2H2O (stable, water soluble salt) is used.
Mechanism of tanning suggests attachement to amino and imino groups.
Ts obtained is up to 100 0C. Tanning time is reported to be 6-9 hours done at acid pHs. Masking agents recommended are citric, tartaric and lactic acids. Acetic, formic and oxalic acids are ineffective. (Knowledge mostly of Soviet origin)
..